EDITORIAL | https://doi.org/10.5005/jp-journals-10078-1280 |
Allostatic Load and Glaucoma: Are We Missing the Big Picture?
1–3Dr RP Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India
Corresponding Author: Tanuj Dada, Dr RP Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India, Phone: +91 11-26593101, e-mail: tanujdada@gmail.com
How to cite this article Dada T, Mahalingam K, Gupta V. Allostatic Load and Glaucoma: Are We Missing the Big Picture? J Curr Glaucoma Pract 2020;14(2):47–49.
Source of support: Nil
Conflict of interest: None
Glaucoma is one of the leading causes of irreversible blindness worldwide,1 and currently lowering of IOP by medical/laser/surgical therapy is the only option for managing glaucoma patients. In general, the focus of ophthalmologists is on the eye and the target IOP in glaucoma patients; however, this approach neglects approaching the disease as a systemic manifestation impacting the eye and treating the patient as a whole.
Both aging and stress can have deleterious effects on glaucomatous optic neuropathy. Stressors are defined as the stimuli that disrupt or threaten to disrupt homeostasis.2 Recently, the concept of allostasis has gained recognition to redefine stress and measure it objectively. According to conceptualization by McEwen and Wingfield, allostasis is the process of maintaining stability (homeostasis) through change in both environmental stimuli and physiological mechanisms.3 Maintenance of physiological parameters in a living organism is homeostasis and the physiological mechanisms that maintain homeostasis is referred to as allostasis.4 On exposure to repeated stressors, there is wear and tear on the organism’s coping mechanisms, and this constitutes what is known as the “allostatic load”. It also indicates how prepared the individual is to cope with future stressors. This concept also proposes a threshold beyond which allostatic load turns into allostatic overload and leads to disease.
Allostatic load is assessed by markers of physiological dysregulation.5 These markers represent four biological systems, such as cardiovascular system, metabolic system, inflammatory system, and neuroendocrine system, and have also been described as consisting of primary mediators and secondary outcomes of stress.6,7 Allostatic load score (ALS) is one of the most well-known measures to quantitate this load. The original description of ALS included 10 parameters: systolic blood pressure, diastolic blood pressure, total cholesterol (TC), high-density lipoprotein (HDL), glycosylated hemoglobin (HbA1c), waist-to-hip ratio (WHR), dehydroepiandrosterone sulfate (DHEA-S), urinary epinephrine, urinary norepinephrine, and urinary cortisol. Each of which is converted into a dichotomous variable, with 1 point given if the biomarker is in the high-risk range (highest or lowest quartile) and 0 if not.8 It has been proposed that neuroendocrine and immunological parameters should be included in allostatic load measurements. There have also been variations in terms of defining the cutoff of each biomarker as well as the way total score is calculated. The total score is usually treated as a continuous variable, and higher scores represent greater allostatic load although few studies have also dichotomized the total score. Currently, there is no accepted “gold standard” measure.9 Some of the biomarkers that have been used in various studies are listed in Table 1.
The risk of mortality increases both with increasing age and an increase in allostatic load. Additionally, the allostatic load also increases with age and stress.10 Stress can modulate the exosomal contents (non-coding RNAs) and accelerate aging, and stress also activates the hypothalamic–pituitary–adrenal (HPA) axis and leads to release of glucocorticoid stress hormones, thereby further increasing the allostatic load.11
INTERPLAY BETWEEN CHRONIC SYSTEMIC DISEASES, ALLOSTATIC LOAD, AND GLAUCOMA
Studies have shown a positive association between allostatic load and hypertension. The drivers of allostatic load have been identified as chronic stressors such as occupational stress, social isolation, marriage and racial discrimination, etc.12,13 Allostatic load also correlates with development of diabetes and hypercholesterolemia.5 In addition, allostatic load has been shown to be associated with other chronic diseases such as abdominal obesity, cardiovascular disease, arthritis, and cancer14 (Flowchart 1).
Older age, diabetes, hypertension, heart disease, and sickle cell anemia are systemic risk factors for glaucoma, and these systemic diseases have been directly linked to the allostatic load. In a study by Dave et al., the prevalence of diabetes mellitus and hypertension was 29.4% and 47.5%, respectively, in patients with glaucoma.15 Other studies report that there is significant association of chronic systemic diseases such as diabetes, hypertension, coronary artery disease, obstructive sleep apnea, etc., with primary glaucoma, but their actual role as a risk factor for glaucoma needs to be further evaluated.16 One possible explanation is that allostatic load is an important risk factor both for systemic diseases and for glaucoma, leading to the observed association between chronic systemic diseases and glaucoma. There are various possible mechanisms by way of which allostatic load may cause or worsen glaucoma (Flowchart 1).
| S. no. | Biomarker | Remarks |
|---|---|---|
| 1 | Urinary cortisol | Primary mediator: Neuroendocrine |
| 2 | Salivary cortisol | Primary mediator: Neuroendocrine |
| 3 | Dehydroepiandrosterone sulfate (DHEA-S) | Primary mediator: Neuroendocrine |
| 4 | Urinary epinephrine | Primary mediator: Neuroendocrine |
| 5 | Urinary norepinephrine | Primary mediator: Neuroendocrine |
| 6 | Heart rate variability | Primary mediator: Neurophysiological |
| 7 | TNF-α | Primary mediator: Anti-inflammatory |
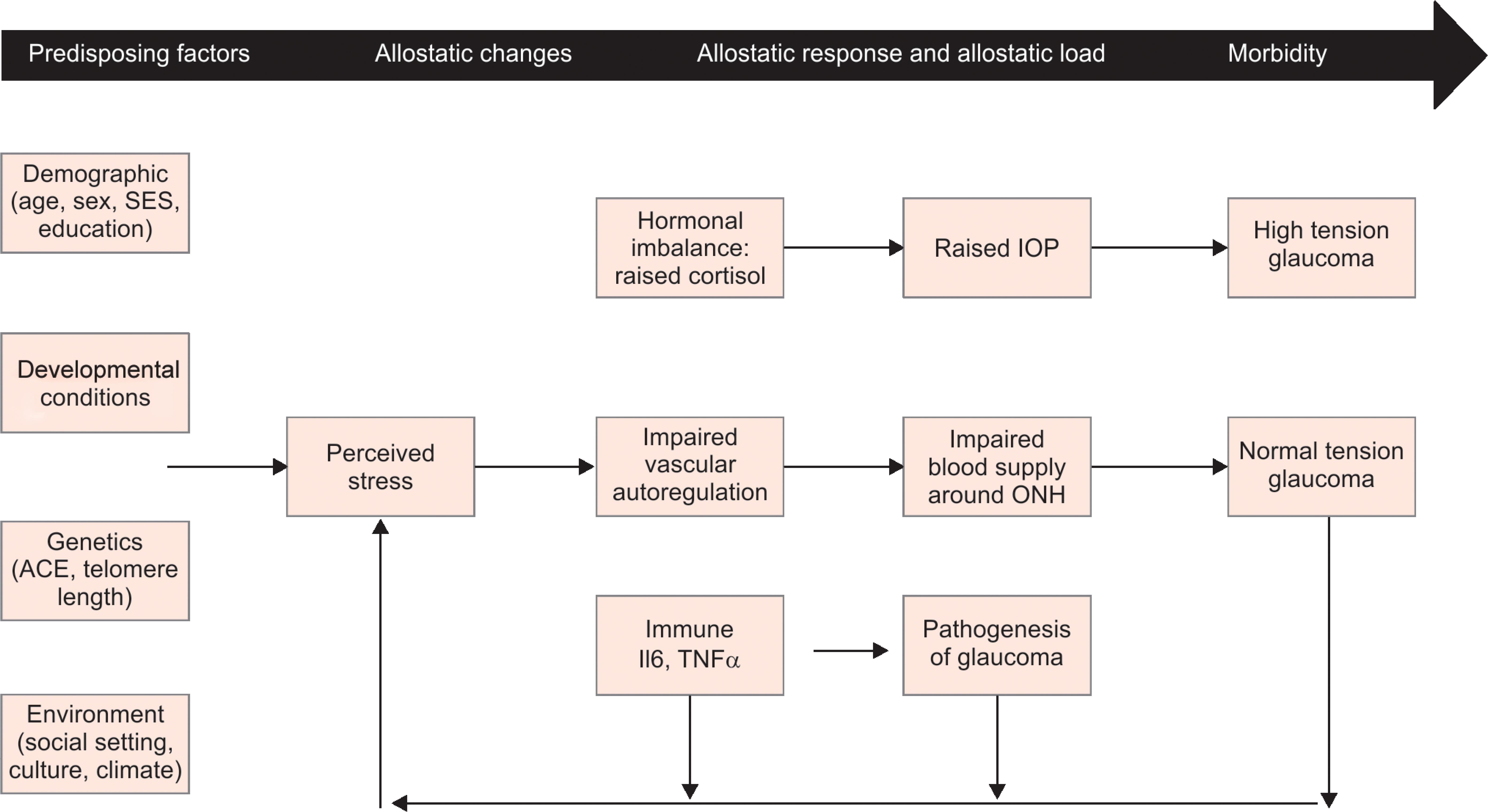
Flowchart 1: Plausible mechanisms of association between allostatic load and glaucoma
Chronic stress can lead to excess secretion of hormones like catecholamines and endogenous glucocorticoids like cortisol. Catecholamines can lead to vasoconstriction and decrease the blood supply to the optic nerve head and retinal ganglion cells, while cortisol can lead to an increase in IOP due to changes in the trabecular meshwork.17 Corticosteroids can also induce myocilin (MYOC) in trabecular meshwork, which is linked to glaucoma.18 The association between allostatic load and the changes it induces in ocular physiology needs our attention and warrants further investigation. Recent studies suggest that stress can cause an increase in intraocular pressure in both open angle and angle closure glaucoma, and techniques of mindfulness and relaxation can decrease it.19–22 Hence, stress can be a causal factor for a rise in intraocular pressure and also be a consequence of it which constitutes a self-perpetuating vicious cycle. Stress and allostatic overload could also be cause of worsening of intraocular pressure in previously controlled patients. Continuous stress with elevated cortisol levels and catecholamines can cause sympathetic nervous system overdrive and vascular dysregulation. This can lead to a decrease in ischemia threshold and contribute to optic neuropathy without rise in IOP (normal pressure glaucoma).23 Thus, stress can lead to high tension glaucoma by raising intraocular pressure through autonomic imbalance/endogenous hypercortisolism and normal tension glaucoma by vascular dysregulation. Stress also provokes chronic inflammation, raising levels TNFα and IL6, and this can be a factor contributing to RGC apoptosis due to neuro-inflammation of the glial cells.24 In general, glaucoma patients suffer from an impaired quality of life which may worsen further with medical/surgical therapy, and this leads to a state of allostatic overload that increases risk of other systemic diseases and mortality.25
THERAPIES TO DECREASE THE ALLOSTATIC LOAD
Interventions that reduce allostatic load may be helpful for patients with glaucoma. In this regard, lifestyle interventions, including a healthy diet, regular aerobic exercises, Yoga, and meditation, may have a significant impact in reducing this load.22,26 A meta-analysis by Pascoe et al. proved that practicing yoga asanas reduced the markers of allostatic load like cortisol, systolic blood pressure, cholesterol, and fasting blood sugar.27 Yoga and meditation has been inculcated as a complete stress reduction program to increase the quality of life and well-being to promote healthy aging.28,29 Meditation was found to be useful in chronic systemic diseases and has shown to improve blood sugars and blood lipid levels in type 2 diabetes mellitus patients.30
Studies have shown that yoga and meditation are helpful in patients with glaucoma, and this effect may be mediated through a reduction of the allostatic load. Meditation may prevent glaucoma progression by reducing IOP through the following mechanisms: decreasing serum cortisol, increasing nitric oxide, augmenting blood flow and brain oxygenation, lowering oxidative stress, and decreasing inflammation.31
Clinically, the association of allostatic load and glaucoma has important implications. After diagnosing a patient with glaucoma, ophthalmologists should make effort to evaluate the allostatic load in conjunction with a physician and suggest dietary/lifestyle modifications that reduce allostatic load, along with the prescribing of glaucoma medications. A team comprising of the glaucoma specialist, psychologist, dietician/fitness expert, and general physician may provide ideal holistic therapy for glaucoma patients. This may prove to be a useful adjunct tool for improving the quality of patients suffering from this irreversibly blinding disease and help us to widen our “target” from lowering IOP to improving the overall health of glaucoma patients.
REFERENCES
1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health 2017;5(12):e1221–e1234. DOI: 10.1016/S2214-109X(17)30393-5.
2. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview Phy Behavior Homeosta JAMA 1992;267(9):1244–1252. DOI: 10.1001/jama.1992.03480090092034.
3. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 2003;43(1):2–15. DOI: 10.1016/S0018-506X(02)00024-7.
4. Romero LM, Dickens MJ, Cyr NE. The reactive scope model - a new model integrating homeostasis, allostasis, and stress. Horm Behav 2009;55(3):375–389. DOI: 10.1016/j.yhbeh.2008.12.009.
5. Rodriquez EJ, Kim EN, Sumner AE, et al. Allostatic load: importance, markers, and score determination in minority and disparity populations. J Urban Health 2019;96 (Suppl 1):3–11. DOI: 10.1007/s11524-019-00345-5.
6. Mauss D, Li J, Schmidt B, et al. Measuring allostatic load in the workforce: a systematic review. Ind Health 2015;53(1):5–20. DOI: 10.2486/indhealth.2014-0122.
7. Duong MT, Bingham BA, Aldana PC, et al. Variation in the calculation of allostatic load score: 21 examples from NHANES. J Racial Ethn Health Disparit 2017;4(3):455–461. DOI: 10.1007/s40615-016-0246-8.
8. Seeman TE, McEwen BS, Rowe JW, et al. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. PNAS 2001;98(8):4770–4775. DOI: 10.1073/pnas.081072698.
9. Mauss D, Jarczok MN, Fischer JE. The streamlined allostatic load index: a replication of study results. Stress 2016;19(6):553–558. DOI: 10.1080/10253890.2016.1219718.
10. Crimmins EM, Johnston M, Hayward M, et al. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol 2003;38(7):731–734. DOI: 10.1016/S0531-5565(03)00099-8.
11. Thomson EM. Air pollution, stress, and allostatic load: linking systemic and central nervous system impacts. J Alzheimer’s Dise 2019;69(3):597–614. DOI: 10.3233/JAD-190015.
12. Spruill TM. Chronic psychosocial stress and hypertension. Curr Hypertens Rep 2010;12(1):10–16. DOI: 10.1007/s11906-009-0084-8.
13. Mocayar Marón FJ, Ferder L, Saraví FD, et al. Hypertension linked to allostatic load: from psychosocial stress to inflammation and mitochondrial dysfunction. Stress 2019;22(2):169–181. DOI: 10.1080/10253890.2018.1542683.
14. Mattei J, Demissie S, Falcon LM, et al. Allostatic load is associated with chronic conditions in the Boston Puerto Rican health study. Soc Sci Med 2010;70(12):1988–1996. DOI: 10.1016/j.socscimed.2010.02.024.
15. Dave A, Bali SJ, Sharma R, et al. Prevalence of diabetes mellitus and hypertension among Indian glaucoma patients and evaluation of systemic therapy. Int Ophthalmol 2013;33(5):527–532. DOI: 10.1007/s10792-013-9737-3.
16. Tham Y-C, Cheng C-Y. Associations between chronic systemic diseases and primary open angle glaucoma: an epidemiological perspective. Clin Experiment Ophthalmol 2017;45(1):24–32. DOI: 10.1111/ceo.12763.
17. Shiels PG, Stenvinkel P, Kooman JP, et al. Circulating markers of ageing and allostatic load: a slow train coming. Pract Lab Med 2016;7:49–54. DOI: 10.1016/j.plabm.2016.04.002.
18. Hoffman EA, Perkumas KM, Highstrom LM, et al. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Invest Ophthalmol Vis Sci 2009;50(3):1313–1318. DOI: 10.1167/iovs.08-2326.
19. Bertelmann T, Strempel I. Short-term effects of relaxation music on patients suffering from primary open-angle glaucoma [internet]. Clinical Ophthalmology, vol. 9. Dove Press; 2015. pp. 1981–1988. Available from: https://www.dovepress.com/short-term-effects-of-relaxation-music-on-patients-suffering-from-prim-peer-reviewed-fulltext-article-OPTH.
20. Jiménez R, Vera J. Effect of examination stress on intraocular pressure in university students. Appl Ergon 2018;67:252–258. DOI: 10.1016/j.apergo.2017.10.010.
21. Gillmann K, Hoskens K, Mansouri K. Acute emotional stress as a trigger for intraocular pressure elevation in Glaucoma. BMC Ophthalmol 2019;19(1):69. DOI: 10.1186/s12886-019-1075-4.
22. Shily BG. Psychophysiological stress, elevated intraocular pressure, and acute closed-angle glaucoma. Am J Optom Physiol Opt 1987;64(11):866–870. DOI: 10.1097/00006324-198711000-00011.
23. Sabel BA, Wang J, Cárdenas-Morales L, et al. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J 2018;9(2):133–160. DOI: 10.1007/s13167-018-0136-8.
24. Quaranta L, Riva I, Gerardi C, et al. Quality of life in glaucoma: a review of the literature. Adv Ther 2016;33(6):959–981. DOI: 10.1007/s12325-016-0333-6.
25. Zheng DD, Christ SL, Lam BL, et al. Visual acuity and increased mortality: the role of allostatic load and functional status. Invest Ophthalmol Vis Sci 2014;55(8):5144–5150. DOI: 10.1167/iovs.14-14202.
26. Villemure C, Ceko M, Cotton VA, et al. Neuroprotective effects of yoga practice: age-, experience-, and frequency-dependent plasticity. Front Hum Neurosci [Internet] 2015. 9. Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2015.00281/full.
27. Pascoe MC, Thompson DR, Ski CF. Yoga, mindfulness-based stress reduction and stress-related physiological measures: a meta-analysis. Psychoneuroendocrinology 2017;86:152–168. DOI: 10.1016/j.psyneuen.2017.08.008.
28. Oken BS, Zajdel D, Kishiyama S, et al. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med 2006;12(1):40–47.
29. Gothe NP, Keswani RK, McAuley E. Yoga practice improves executive function by attenuating stress levels. Biol Psychol 2016;121(Pt A):109–116. DOI: 10.1016/j.biopsycho.2016.10.010.
30. Xia T, Yang Y, Li W, et al. Meditative movements for patients with type 2 diabetes: a systematic review and meta-analysis. Evidence-based Complement Alternat Med: eCAM 2020;2020:5745013. DOI: 10.1155/2020/5745013.
31. Dada T, Ramesh P, Shakrawal J. Meditation: a Polypill for comprehensive management of glaucoma patients. J Glaucoma 2020;29(2):133–140.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.